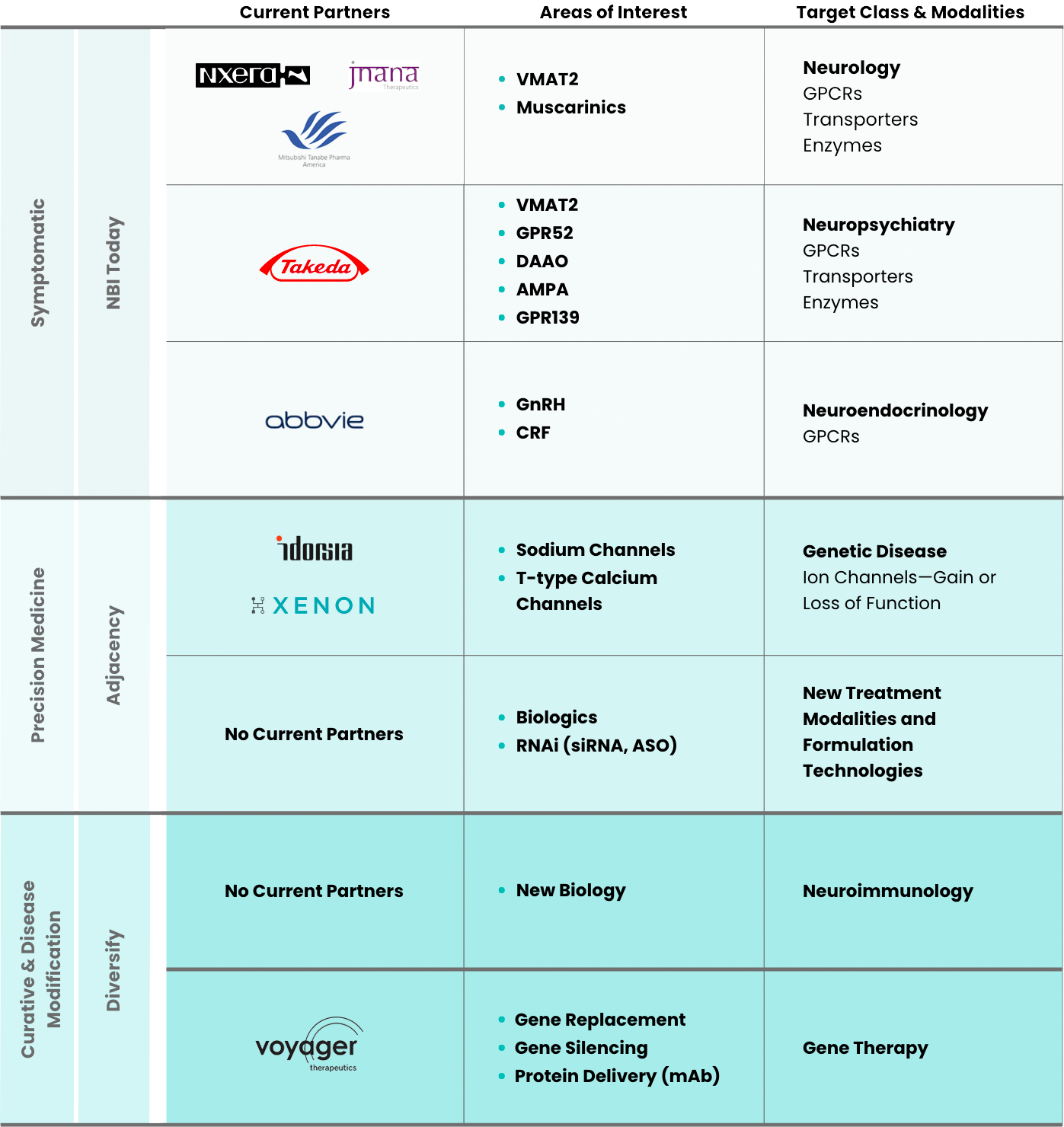
AbbVie
In June 2010, Neurocrine Biosciences entered into an exclusive worldwide collaboration with AbbVie (formerly Abbott) to develop and commercialize elagolix for women’s health. Under the terms of the agreement, AbbVie is responsible for future development and commercialization.
In 2018, AbbVie received U.S. Food and Drug Administration approval for ORILISSA® (elagolix) to treat pain associated with endometriosis. In 2020, AbbVie received U.S. Food and Drug Administration approval for ORIAHNN™ (elagolix, estradiol, and norethindrone acetate capsules; elagolix capsules) to manage heavy menstrual bleeding associated with uterine fibroids in pre-menopausal women. Under the collaboration, elagolix is also being investigated for the treatment of polycystic ovary syndrome. AbbVie is responsible for all development, marketing, and commercialization costs, and Neurocrine Biosciences is entitled to a royalty on worldwide sales of ORILISSA, ORIAHNN, and any other product containing elagolix.
Mitsubishi Tanabe Pharma
In March 2015, Neurocrine Biosciences entered into an exclusive collaboration and licensing agreement with Mitsubishi Tanabe Pharma for the development and commercialization of valbenazine in Japan and other select Asian markets.
Mitsubishi Tanabe Pharma will be responsible for all development, marketing, and commercialization costs in their territories, and Neurocrine Biosciences will be entitled to a percentage of sales by Mitsubishi Tanabe Pharma. Neurocrine Biosciences retains full commercial rights to valbenazine in North America, Europe, and other countries outside of Asia.
Jnana Therapeautics
In October 2018, Neurocrine Biosciences entered into a research collaboration with Jnana Therapeutics to discover novel small molecule therapeutics for multiple targets associated with central nervous system (CNS) disorders. The collaboration leverages Jnana’s proprietary drug discovery platform across the solute carrier (SLC) family of transporters and Neurocrine Biosciences’ research and development expertise in CNS disorders to advance new medicines.
Voyager Therapeutics
In January 2019, Neurocrine Biosciences entered into a strategic collaboration with Voyager Therapeutics focused on the development and commercialization of Voyager Therapeautics’ gene therapy programs, including VY-FXN01 for Friedreich’s ataxia, as well as rights to two discovery programs.
Xenon Pharmaceuticals Inc.
In December 2019, Neurocrine Biosciences entered into a license and collaboration agreement with Xenon Pharmaceuticals to identify, research, and develop sodium channel inhibitors, including clinical candidate NBI-921352 and three preclinical candidates, which it will have the exclusive right to further develop and commercialize. NBI-921352 is a potent, highly selective sodium channel inhibitor (Nav1.6) being developed to treat pediatric patients with SCN8A-DEE and other potential indications.
Idorsia Ltd.
Neurocrine Biosciences acquired the global rights to NBI-827104 from Idorsia in May 2020. NBI-827104 is an investigational, potent, selective, orally active, brain penetrating T-type calcium channel blocker (Cav 3.1, Cav 3.2, Cav 3.3) for the potential treatment of epileptic encephalopathy with continuous spike-and-wave during sleep (EE-CSWS). We are also developing NBI-827104 for the potential treatment of essential tremor. The agreement also includes a research collaboration to discover and identify additional novel T-type calcium channel blockers as development candidates.
Takeda Pharmaceutical Company Ltd.
In June 2020, Neurocrine Biosciences entered into a strategic collaboration with Takeda to develop and commercialize compounds in Takeda’s early-to-mid-stage psychiatry pipeline. Specifically, Neurocrine Biosciences received an exclusive license for seven Takeda pipeline programs, including clinical stage assets, which we are studying in schizophrenia and treatment-resistant depression.
Nxera Pharma (formerly Sosei Heptares)
In December 2021, Neurocrine Biosciences entered into a strategic collaboration and licensing agreement with Nxera Pharma (formerly Sosei Heptares) to develop novel muscarinic receptor agonists, which Neurocrine Biosciences intends to study for the treatment of schizophrenia, dementia, and other neuropsychiatric disorders.
Contact us about partnership opportunities at bd@neurocrine.com